EBSD Explained
Techniques
Applications
Hints and Tips
Technology
OXFORD INSTRUMENTS EBSD PRODUCTS
CMOS Detector RangeAZtecHKL Acquisition SoftwareAZtecCrystal Processing Software
Directly polished surfaces can be inspected using Electron Backscatter Diffraction (EBSD), but in many cases, if the sample is conductive, the pattern quality can be improved by electrolytic polishing.
Electrolytic preparation uses an electrolytic reaction cell containing a liquid electrolyte with two electrodes: an anode and cathode. The sample to be polished / etched forms the anode. Current is applied which forces the metal of the anode to dissolve, to wander and deposit itself on the cathode as a coating. The electrodes are connected to an external power supply and voltage applied to cause reaction within the cell.
For EBSD of metallic samples only electrolytic polishing is usually adequate, electrolytic etching is usually not necessary.
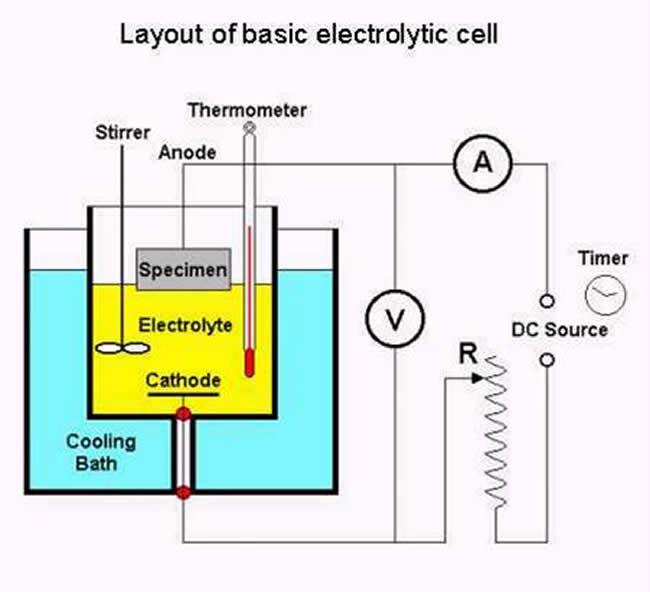
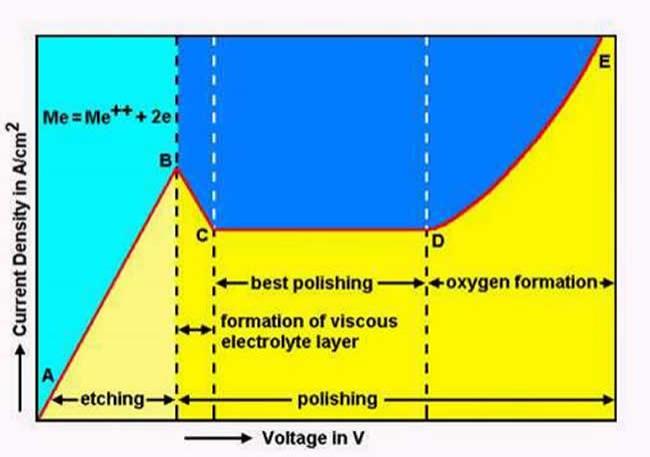
Shown above is the characteristic curve for an electrolytic cell. This curve is dependent on the electrolyte used and will vary for different electrolytes. Control of the voltage and current density at the anode, plus electrolyte composition, temperature and agitation are all critical in achieving the desired polishing/etching characteristics. Establishing adequate control of these parameters can be difficult and further, many of the electrolytes are hazardous or even explosive. In the case of the latter, temperature control is critical. Do not attempt electrolytic polishing or etching without the necessary experience and safety measures in place.
Factors controlling etching/polishing characteristics include:
| Advantages: | Disadvantages: |
| Polishing and etching are possible | Conductive specimens only |
| Fast preparation process | Not all alloys can be polished |
| Is reproducible when the process is automated | Preferential attack or pitting can occur |
| No mechanical deformation and smeared surfaces | No edge retention |
| Can produce excellent surfaces for EBSD | Limited polishing area |
| Limited scratch/material removal | |
| Hazardous Electrolytes | |
| Temperature control vital |
Directly polished surfaces can be inspected using EBSD, but in many cases the pattern quality is improved by etching. Additionally, etching delineates the grain structure, which is of obvious benefit. However, etching may attack a second phase preferentially, or attack grain boundaries excessively. Caution should be exercised when choosing and using etchants. Inspect the sample surface using a light microscope before and after etching to assess the effect. Materials that are difficult to polish may benefit from repeated etching and re-polishing. This method can expose an undamaged surface suitable for EBSD when conventional polishing and etching fails to achieve an adequate surface. Using special acid or alkali resistant cloths, it is also possible to add dilute etchants to the polishing cloth during polishing. This can be effective but can be difficult to control. Some experience is required.
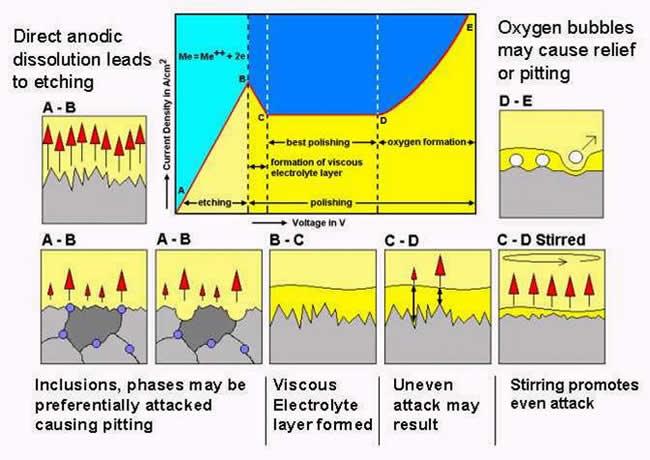
Any etchant that is used must dissolve the specimen surface in an even manner, and not leave behind any oxide or reaction product layers. Such layers can completely suppress diffraction. Many etchants listed in metallographic textbooks are 'contrast etches' which rely on the formation of different thickness oxide layers to generate colours visible using a light microscope. Therefore such etchants are generally not suitable for EBSD.